Why Are Hybridization Orbital Useful When Describing Molecules
You can assign it some degree of reality for argumentive purposes. Chemistry Molecular Orbital Theory Orbital Hybridization.

Orbital Hybridisation Wikiwand
Sp hybridization is also called diagonal hybridization.
. For example Werner Kutzelnigg published a couple of important papers in the 1980s arguing that hybridization is less important for elements in the third row because theres a larger difference in size and energy between s and p orbitals in those atoms. Describe the orbital hybridization around the central atom in NH2-. In CH4 the bond angle is 1095.
Hybridized orbitals are very useful in explaining of the shape of molecular orbitals for molecules. Evaporation is counted as surface phenomenon that results in the conversion of liquid molecules into. The orientation of these orbitals determines the geometry of the molecule.
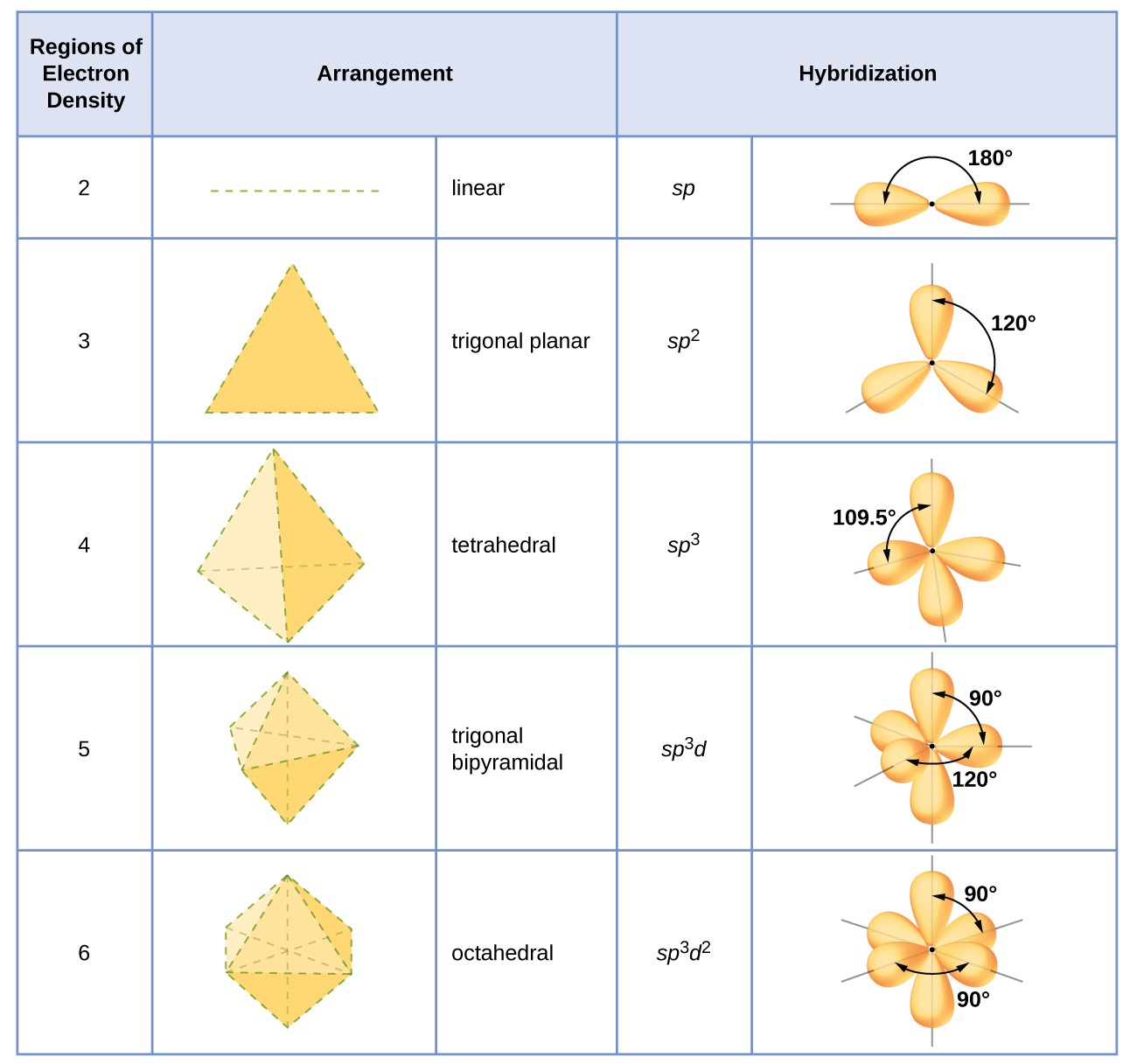
VSEPR Theory predicts the geometry and chemists use hybridization to explain it. It is an expansion of the valence bond theory. 2p_x 2p_y and 2p_z Explanation.
Chemists use hybridization to explain molecular geometry. Orbital hybridization is a mathematical manipulation of atomic wavefunctions but that that is the only relationship to a quantum mechanical description of an atom. For example we can use orbital hybridization to understand why methane has the formula CH₄ how the overlap of carbon and hydrogen atomic orbitals is used to form electron-pair bonds and why the arrangement of the C-H bonds about the central carbon is tetrahedral.
And these three. Hybridization is the formation of hybrid orbitals by mixing two or more atomic orbitals. Explain what a hybrid orbital is.
Its a very simple and useful approximation that allows us to make a lot of accurate predictions about the shapes of molecules and the way they react but there isnt anything true about hybridization. But hybridization works only for elements in the second period of the Periodic Table and best for carbon. Describe the orbital hybridization around the central atom in NH2-.
Hybridization theory is a technique we use to describe the orbital structure of a molecule. Orbital hybridization provides information about both molecular bonding and molecular shape. Explain why atomic orbitals are not sufficient to describe bonding in molecules.
Molecular orbital approach based on linear combination of atomic orbitals bonding and anti bonding orbitals Hybridization is based on mixing of orbitals of the atom any to give hybrid orbitalsHybrid orbitals can overlap with the hybridpure orbitals of other atoms to give sigma bonds. Sp2 3sigma bond1 pi bond sp3 4 sigma bond. Determine number of sigma and pi bonds in.
In what ways is orbital hybridization useful in describing molecules. Part 1 Describe the hybridization type molecular shape and bond angle in each of these molecules. However hybrid orbitals and pure atomic orbitals have different.
Orbital hybridization can determine how many bonds an atom can form and the shape of molecules. For example using the Aufbau principle Hunds rule and the Pauli exclusion principle we would write the following electron configuration for carbon 1s2 2s2 2p2. Modern valence bond theory has been used to enforce sp 3 d 2 hybridization in SF 6.
PFS Part 2 Using orbital diagrams show how sp hybrid orbitalls are formed from 2s and 2p orbitals in carbon Part 3 Using Molecular Orbital Theory explain why diatomic oxygen O2 contains both. Orbital hybridization is useful because it provides information about both molecular bonding and molecular shape. Hybridization occurs only at some stage in the bond formation and not in an isolated gaseous atom.
Understanding the hybridization of different atoms in a molecule is important in organic chemistry for understanding. Bonding theory is one of the important concepts in chemistry because it provides an explanation on how atoms bond together for the formation of complex. Hybridized orbitals are useful in explaining the shapes of and types of bonding in molecules.
In order to explain the three-dimensional shape of molecules scientists use valence-shell electron-pair repulsion theory VSEPR theory. How do we describe the orbital hybridization in the water molecule. We can therefore use a molecular orbital energy-level diagram and the calculated bond order to predict the relative stability of species such as H2.
The form of the molecule may be predicted if the hybridization of the molecule is understood. It forms linear molecules with an angle of 180 This type of hybridization involves the mixing of one s orbital and one p orbital of equal energy to give a new hybrid orbital known as an sp hybridized orbital. When thinking of chemical bonds atoms do not use atomic orbitals to make bonds but rather what are called hybrid orbitals.
I find orbital hybridization of very limited use. Alternative bonding schemes with no d-orbital contribution are presented and yield lower total energies in. My best understanding is that orbital hybridization is more of a conceptual tool used to understand bonding properties in molecules.
Presently we have no evidence that supports the claim that orbital hybridization happens. Molecular orbital theory is true the electrons dont exist in hybridized orbitals they exist in molecular orbitals spread across the entire. Explain the difference between a sigma and pi bond with respect to the overlap of orbitals to create bonds.
Determine the hybridization of a substance based on its dot diagram. In chemistry hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for describing bonding properties. The bigger lobe of the hybrid orbital constantly has a high-quality sign at the same time as the smaller lobe on the other facet has a bad sign.
Why are bonding theories so important. 1 Answer anor277 Aug 9 2017 The oxygen atom has 3xxp-orbitals ie. Hybridization is a model we use to describe bonding.
I never managed to gather a sense of orbital merging as an actual event because an orbital itself is just a description of the location of electron density in a bond. Hybridization is the process of mixing two or more atomic orbitals to create new covalently bonded orbitals in molecules.
Why Do Orbitals Hybridize Does Hybridization Occur In Every Molecule Quora
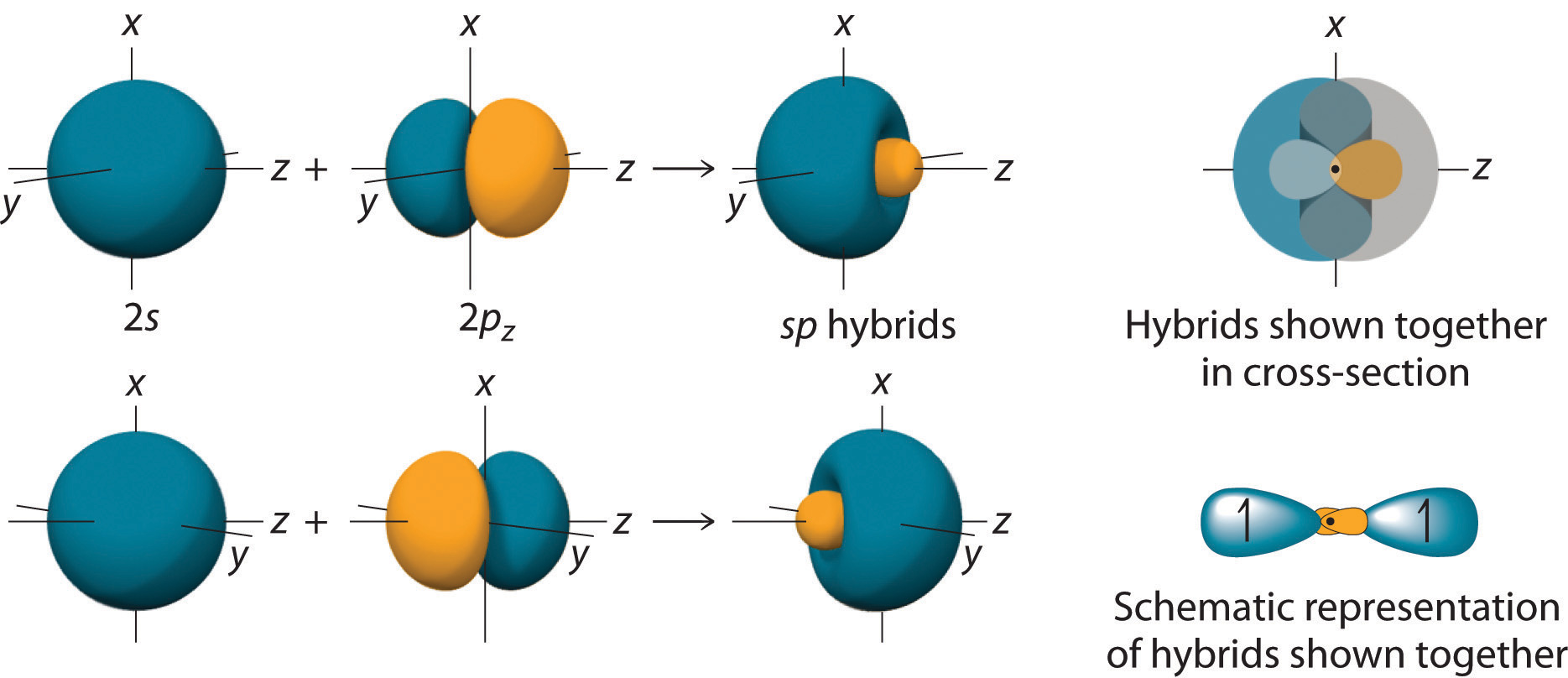
10 7 Valence Bond Theory Hybridization Of Atomic Orbitals Chemistry Libretexts
Comments
Post a Comment